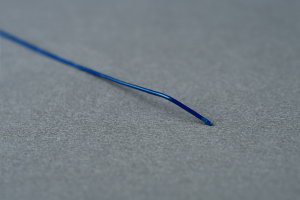
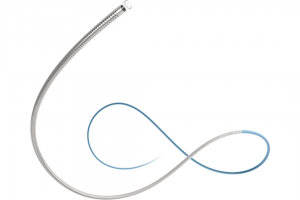
Coil Delivery Microcatheter Neuro Intervention coil
● Product Characteristics
·The semi-rigid proximal section transitions to a flexible distal tip to facilitate advancement through vessels. Dual radiopaque markers at the distal end facilitate fluoroscopic visualization.The outer slirface of the catheter is coated to increase lubricity.A luer fitting on the Microcatheter hub is used for the attachment of accessories.
·The Microcatheter is suitable for professional to selectively inject or input control medium and/or liquid and/or embolic meterials, and/or appropriate devices (such as stents ,coils) to the periphery and neuro vasculature.
● Product Use
The Microcatheter is intended for professional physicians to selectively inject or infuse control media and/or liquids and/or embolic materials, and/or appropriate devices (such as stents, coils) into peripheral and neurovascular.
● Product Parameter
|
Model |
Specification |
Proximal OD(Fr/mm) |
Distal OD(Fr/mm) |
ID(in/mm) |
length (cm) |
Tip configuration |
|
1.7F |
HMC-17-150 |
2.1/0.68 |
1.7/0.57 |
0.017/0.43 |
150 |
Straight |
|
HMC-17-150-45 |
2.1/0.68 |
1.7/0.57 |
0.017/0.43 |
150 |
45° |
|
|
HMC-17-150-90 |
2.1/0.68 |
1.7/0.57 |
0.017/0.43 |
150 |
90° |
|
|
2.0F |
HMC-20-150 |
2.4/0.78 |
2.0/0.67 |
0.017/0.43 |
150 |
Straight |
|
HMC-20-150-45 |
2.4/0.78 |
2.0/0.67 |
0.017/0.43 |
150 |
45° |
|
|
HMC-20-150-90 |
2.4/0.78 |
2.0/0.67 |
0.017/0.43 |
150 |
90° |
● About Us
Zhuhai Ton-Bridge Medical Technology Co., Ltd. It is a high-tech enterprise with a registered capital of 230 million yuan. We are engaged in independent research and development, achievement transformation and industrialization of medical devices for neurovascular implantation and intervention.
The company has successfully transformed a number of advanced scientific research achievements into products, promoting the development of domestic intracranial implant interventional medical devices. Ton-Bridge Medical has obtained 7 NMPA Class III medical device registration certificates, 1 NMPA Class II medical device registration certificate and 3 EU CE certificates, and has passed ISO9001 quality management system certification and ISO13485 quality management system certification for medical devices.